Chemical Bonds: Ionic, Covalent, and Metallic Bonding in Biochemistry
Chemical bonds are the essential forces that dictate how atoms combine to form the diverse array of molecules and compounds that constitute our world. These bonds arise from the interactions of valence electrons as atoms seek a stable state with a full outer shell, often adhering to the octet rule. This stability can be reached through the complete transfer of electrons, leading to the formation of ionic bonds or by sharing electrons, creating covalent bonds. The nature of the chemical bond is dependent on the electronegativity differences between the atoms involved, influencing the strength, directionality, and overall characteristics of the resulting compounds.
Understanding the nuances of these bonds is critical to grasping the properties of matter, from the hardness of diamonds to the flexibility of organic molecules.
The Drive for Chemical Bonds: Stability and the Octet Rule
- Core Idea: Atoms bond to achieve a more stable, lower energy state. Individual, unbonded atoms are typically not in their most stable arrangement.
- Valence Shell: Stability is achieved when an atom’s outermost shell, the valence shell, is filled with electrons. This means having eight electrons (the octet rule) for most atoms. Hydrogen and helium are exceptions, requiring only two electrons.
- Atoms want to reach the most stable or lowest energy state they can…atoms are stable when their outermost, called their valence shell, is filled with electrons.
- How to Fill the Shell: Atoms achieve an entire valence shell by losing, gaining, or sharing electrons with other atoms.
Types of Strong Chemical Bonds
General Principle: Strong chemical bonds involve the transfer or sharing of electrons, relying on the electrostatic attraction between positively charged nuclei and negatively charged electrons. The type of bond formed depends on the difference in electronegativity between the atoms involved.
Electronegativity: The tendency of an atom to attract shared electrons. A large electronegativity difference results in a more polar (ionic) bond.
A strong chemical bond is formed from the transfer or sharing of electrons between atomic centers. It relies on the electrostatic attraction between the protons in nuclei and the electrons in the orbitals. The types of strong bonds differ due to the difference in electronegativity of the constituent elements.
Ionic Bonds
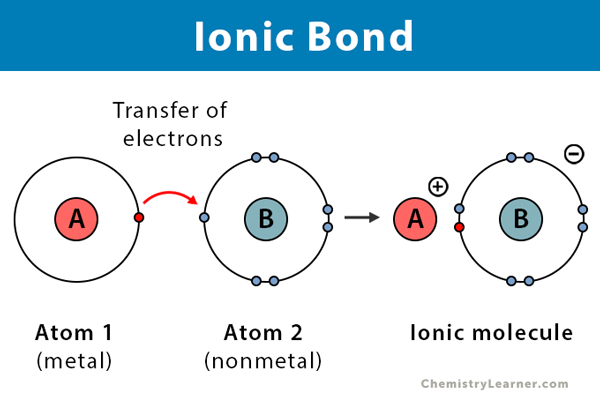
Mechanism: Transfer of electrons from a metal to a non-metal. The metal becomes a positively charged ion (cation), and the non-metal becomes a negatively charged ion (anion).
Electrostatic Attraction: The bond results from the attraction between these oppositely charged ions.
Crystal Lattice: Ionic compounds are not individual units but form repeating three-dimensional crystal structures called crystal lattices.
Example: Table salt (sodium chloride, NaCl). Sodium (metal) loses one electron to chlorine (non-metal).
In an ionic bond, electrons are transferred from a metal to a non-metal, creating oppositely charged ions. The electrostatic attraction that occurs between these oppositely charged ions is called an ionic bond
Properties: Ionic bonds are strong but brittle, with high melting points. They lack specific direction in space.
Covalent Bonds
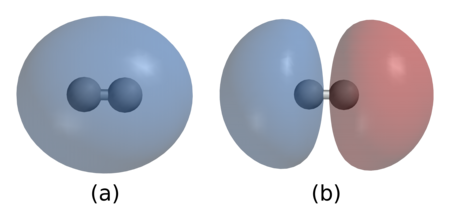
In (a), the two nuclei are surrounded by a cloud of two electrons in the bonding orbital that holds the molecule together. (b) shows hydrogen’s antibonding orbital, which is higher in energy and usually is not occupied by any electrons. (From Wikipedia, Chemical Bonds)
Mechanism: Sharing of electrons between atoms, primarily non-metals.
Formation: Shared electron pairs form a new orbital extending around the nuclei of both atoms, creating a molecule.
- Nonpolar Covalent Bonds: Electrons are shared equally (or very nearly equally) when the electronegativity values are similar or very similar. The shared electrons spend equal time around each atom. Example: H2. When electrons are shared evenly, it’s called a nonpolar covalent bond.
- Polar Covalent Bonds: Electrons are shared unequally due to differing electronegativity. This creates a partial negative charge on the more electronegative atom and a partial positive charge on the less electronegative atom. Example: HCl. When the electrons are shared unequally ,it’s called a polar covalent bond.
Bond Multiplicity: Covalent bonds can be single, double, or triple, depending on the number of shared electron pairs.
Directionality: Covalent bonds have direction in space and can be represented by lines in drawings.
Specific Numbers: Different atoms form specific numbers of covalent bonds: hydrogen (1), oxygen (2), nitrogen (3), and carbon (4).
Molecules: Covalent bonds often result in molecules.
Metallic Bonds
Mechanism: Metal atoms donate electrons to a “sea” of delocalized electrons shared between many atoms.
Bonding: Results from the attraction between positively charged metal ions and the delocalized electrons.
Properties: Strong bonds but more collective, which results in malleability, good electrical and thermal conductivity, and metallic luster.
Differences Between Ionic and Covalent Chemical Bonds
Here’s a table summarizing the key differences between ionic and covalent bonds:
| Feature | Ionic Bond | Covalent Bond |
| Electron Behavior | Transfer of electrons from a metal to a non-metal | Sharing of electrons between non-metal atoms |
| Electronegativity Difference | Large difference in electronegativity (typically > 1.7) | Small to moderate difference in electronegativity (0-1.7) |
| Bond Formation | Electrostatic attraction between oppositely charged ions (cations and anions) | Sharing of electron pairs between atoms |
| Directionality | Generally non-directional | Directional |
| Structure | Forms repeating three-dimensional crystal lattices | Forms molecules or extended networks |
| Types of Compounds | Typically forms ionic compounds, such as salts (e.g., NaCl) | Typically forms molecules, polymers (e.g., nylon), or solids (e.g., diamond, quartz) |
| Strength | It can vary in strength. Strong within molecules but weaker between molecules | It can be strong and tough (e.g., diamond), or soft (e.g., oils and waxes) |
| Brittleness | Brittle, due to short-range forces that do not easily bridge cracks | It can be strong and tough (e.g., diamond) or soft (e.g., oils and waxes) |
| Polarity | Usually forms ions with a full positive or negative charge | Can form polar covalent bonds with partial charges or nonpolar bonds with no charge |
| Examples | Sodium chloride (NaCl), sodium cyanide (NaCN) | Hydrogen molecule (H2), methane (CH4), hydrogen chloride (HCl). |
Additional points on Covalent Bonds
- Nonpolar Covalent Bonds occur when atoms have the same or similar electronegativity values, resulting in an equal sharing of electrons.
- Polar Covalent Bonds: These occur when atoms have moderately different electronegativity values (0.3 to 1.7), resulting in an unequal sharing of electrons, which creates partial charges.
Weak Bonds (Intermolecular Forces)
- Intermolecular Forces: Weaker forces of attraction and repulsion between molecules not covalently bonded.
- Van der Waals Forces: Interactions between closed-shell molecules. These include:
- Keesom forces: Forces between permanent dipoles of polar molecules.
- London dispersion forces: Forces between induced dipoles.
- Hydrogen Bonds: A special type of dipole-dipole interaction that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom (N, O, or F) and then weakly interacts with another electronegative atom in a different molecule.

Historical Context and Theories of Chemical Bonding
- Early Concepts: Early ideas involved chemical affinity and “forces” between atoms.
- Newton: Atoms attract one another through powerful force at minimal distances.
- Berzelius: Developed a theory of chemical combination stressing electronegative and electropositive characters of atoms.
- Valency: The idea of “combining power” of atoms.
- Lewis and Kossel: * Lewis: Concept of electron-pair bonds (sharing) * Kossel: Electron transfers between atoms to explain ionic bonds.
- Bohr: Electrons of atoms form a rotating ring between the nuclei.
- Quantum Mechanics: Quantum approach to chemical bonds could be correct (Burrau 1927).
- Heitler and London developed valence bond theory, which considers electron pairs localized and shared by two atoms.
- Molecular orbital theory considers electrons as delocalized in orbitals that extend throughout the molecule.
Representation of Bonds
- Complexity: Atoms and molecules are three-dimensional, so representing them requires different methods.
- Molecular Formulas: Bonds are indicated differently based on the type of discussion.
- Variations: Representation can vary from full two-dimensional to compressed, showing only functional groups.
Bond Lengths and Energies
- Tables: The document provides a table with typical bond lengths (in picometers) and bond energies (in kilojoules per mole) for various bonds. Different bonds have different lengths and energies, which correlates with the strength of the bond.
| Bond | Length (pm) | Energy (kJ/mol) |
|---|---|---|
| H — Hydrogen | ||
| H–H | 74 | 436 |
| H–O | 96 | 467 |
| H–F | 92 | 568 |
| H–Cl | 127 | 432 |
| C — Carbon | ||
| C–H | 109 | 413 |
| C–C | 154 | 347 |
| C–C= | 151 | |
| =C–C≡ | 147 | |
| =C–C= | 148 | |
| C=C | 134 | 614 |
| C≡C | 120 | 839 |
| C–N | 147 | 308 |
| C–O | 143 | 358 |
| C=O | 745 | |
| C≡O | 1,072 | |
| C–F | 134 | 488 |
| C–Cl | 177 | 330 |
| N — Nitrogen | ||
| N–H | 101 | 391 |
| N–N | 145 | 170 |
| N≡N | 110 | 945 |
| O — Oxygen | ||
| O–O | 148 | 146 |
| O=O | 121 | 495 |
| F, Cl, Br, I — Halogens | ||
| F–F | 142 | 158 |
| Cl–Cl | 199 | 243 |
| Br–H | 141 | 366 |
| Br–Br | 228 | 193 |
| I–H | 161 | 298 |
| I–I | 267 | 151 |
Key Takeaways
- Chemical bonding is essential for forming molecules and compounds.
- Atoms bond to achieve stability by filling their valence shells.
- There are three main types of strong bonds: ionic, covalent, and metallic, each with unique characteristics and formation mechanisms.
- Intermolecular forces are essential for explaining physical properties.
- Understanding bonding is critical to understanding the structure and behavior of matter.
Chemical Bonds: Frequently Asked Questions (FAQs)
Why do atoms form chemical bonds instead of existing independently?
Atoms bond with each other to achieve the most stable, lowest energy state possible. Most atoms have an incomplete valence shell (the outermost shell of electrons), and therefore are not in their most stable arrangement. They will lose, gain, or share electrons with other atoms through chemical bonding to achieve a full valence shell, representing a lower energy state and thus, stability. For many atoms, a full valence shell means having eight electrons, known as the octet rule, though hydrogen and helium only need two.
What are the two main types of strong chemical bonds and how do they differ?
The two main strong chemical bonds are ionic and covalent bonds. Ionic bonds occur between a metal and a non-metal, transferring electrons from one atom to another, creating positively and negatively charged ions. The attraction between these oppositely charged ions is the ionic bond. Covalent bonds, on the other hand, happen when atoms (usually non-metals) share electrons. In a covalent bond, electrons are shared between the nuclei of the atoms.
How is an ionic bond formed?
An ionic bond is formed by transferring electrons from a metal atom to a non-metal atom. Metals tend to have a few loosely held electrons in their outer shells. In contrast, non-metals tend to have nearly full outer shells and exert a stronger pull on electrons (higher electronegativity). This leads to the metal atom losing electrons and becoming a positively charged ion (cation) and the non-metal gaining electrons and becoming a negatively charged ion (anion). The electrostatic attraction between the cation and anion is the ionic bond. Ionic compounds tend to form crystal lattices rather than individual units.
What are the different types of covalent bonds?
Covalent bonds can be nonpolar or polar. A nonpolar covalent bond occurs when electrons are shared equally between two atoms, usually because the atoms have similar electronegativity. A polar covalent bond happens when atoms share electrons unequally due to differences in electronegativity.
This results in a partial negative charge on the more electronegative atom and a partial positive charge on the less electronegative atom, creating a dipole. Covalent bonds can also be single, double, or triple, depending on how many electron pairs are shared between the atoms.
What is the octet rule, and are there any exceptions? The octet rule states that many atoms are most stable when they have eight electrons in their valence shell. However, some notable exceptions exist, such as hydrogen and helium, which only require two electrons to fill their valence shells.
What are metallic bonds, and how are they different from ionic and covalent bonds?
Metallic bonds occur in metals where electrons become delocalized, forming a “sea” of electrons shared among many metal atoms. These delocalized electrons allow for good electrical and thermal conductivity and the characteristic metallic luster. Unlike ionic bonds, where electrons are transferred, and covalent bonds, where electrons are localized between two atoms, metallic bonds involve many atoms collectively sharing many electrons. This allows metals to be malleable and ductile.
What are intermolecular forces, and how do they differ from intramolecular forces?
Intramolecular forces are the strong chemical bonds (ionic, covalent, metallic) that hold atoms together within a molecule or a crystal. Intermolecular forces are weaker attractions or repulsions that occur between molecules that are not covalently bonded. These forces influence physical properties like melting and boiling points. Examples include Van der Waals forces (Keesom, London dispersion), and hydrogen bonding.
How has our understanding of chemical bonds developed over time?
Early ideas of chemical bonding included vague concepts of “affinity” or “force”. Later developments highlighted the role of electronegativity. The modern understanding of chemical bonds evolved through the work of scientists like Rutherford, Lewis, Kossel, and Bohr. The development of quantum mechanics was a key development, leading to valence bond theory and molecular orbital theory, allowing for a more detailed understanding of how electrons are shared or transferred and how the spatial properties of bonds work. These models are still being used and refined today.
Glossary of Terms for Chemical Bonds
- Anion: A negatively charged ion formed when an atom gains electrons.
- Antibonding Orbital: A molecular orbital that weakens the bond between atoms when occupied.
- Bond Length: The distance between the nuclei of two bonded atoms.
- Bonding Orbital: A molecular orbital that strengthens the bond between atoms when occupied.
- Cation: A positively charged ion formed when an atom loses electrons.
- Chemical Bond: The attractive force that holds atoms together in molecules, crystals, and other structures.
- Coordinate Covalent Bond (Dipolar Bond): A covalent bond where both electrons are donated by one atom.
- Covalent Bond: A chemical bond formed by the sharing of electrons between atoms.
- Crystal Lattice: A repeating three-dimensional arrangement of atoms or ions in a crystalline solid.
- Delocalized Electrons: Electrons that are not associated with a single atom or bond but are shared across multiple atoms, as seen in metallic bonding.
- Dipole-Dipole Interactions: Intermolecular forces between polar molecules.
- Electronegativity: The tendency of an atom to attract shared electrons in a chemical bond.
- Intermolecular Forces: Weak forces of attraction or repulsion between molecules, influencing their physical properties.
- Intramolecular Forces: Forces within a molecule, typically referring to chemical bonds.
- Ionic Bond: A chemical bond formed by the electrostatic attraction between oppositely charged ions, typically from the transfer of electrons between metals and nonmetals.
- London Dispersion Forces: Weak intermolecular forces resulting from temporary dipoles in molecules.
- Metallic Bond: A chemical bond formed between metal atoms due to delocalized electrons.
- Molecular Orbital Theory: A theory of chemical bonding that describes bonds as combinations of atomic orbitals.
- Molecule: A group of two or more atoms held together by covalent bonds.
- Nonpolar Covalent Bond: A covalent bond in which electrons are shared equally.
- Octet Rule: The principle that many atoms achieve stability by having eight electrons in their outermost valence shell.
- Polar Covalent Bond: A covalent bond where electrons are shared unequally, creating partial charges.
- Valence Electrons: The electrons in the outermost shell of an atom, which are involved in chemical bonding.
- Valence Bond Theory: A theory of chemical bonding that describes bonds as resulting from the overlap of atomic orbitals.